Rusan Pharma’s API Plant in Ankleshwar (India)Receives USFDA GMP Approval
RUSAN PHARMA PRIVATE LIMITED,a pharmaceutical company based in India specializing in the area of addiction treatment and pain management, recently announced that the United States Food and Drug Administration (US FDA) has granted Good Manufacturing Practice (GMP) approval for its…
SOL LABS Introduces Two New Innovative Sun Protection Solutions
SOL LABS, a pioneering name in sun care, proudly announces the launch of two groundbreaking products designed to redefine sun protection on-the-go and elevate eco-conscious practices in skincare. Building upon the success of the brand’s hero skus, the Natural Mineral…
Beckman Coulter’s DxI 9000 Immunoassay Analyzer Honored at Premier, Inc.’s Annual Supplier Innovation Celebration
Beckman Coulter Diagnostics, a clinical diagnostics leader, today announced that the DxI 9000 Immunoassay Analyzer* was honored during Premier, Inc.’s annual supplier Innovation Celebration at its 2024 Breakthroughs Conference and Exhibition. The Innovation Celebration recognizes groundbreaking healthcare technologies that have been…
Aspen Laser Launches New Ascent Laser Series
Aspen Laser Systems, LLC, an emerging global leader in the medical device industry, with a focus on photobiomodulation, announced the official launch of the Ascent Laser Series. The new therapeutic laser series features 32 watts of combined power and the…
Soterix Medical Announces FDA 510(k) Clearance of MEGA-IOM Intraoperative Neuromonitoring System
Soterix Medical Inc., the global leader in stimulation and synergistic monitoring technologies, announced today it has received a 510(k) clearance from the U.S. Food & Drug Administration for its Intraoperative Neurophysiologic Monitoring (IOM) system, MEGA-IOM. The system provides unmatched integrated control…
Beacon Biosignals Announces Strategic Collaboration with Skye Bioscience to Integrate Sleep Quality and Sleep Apnea Endpoints in CBEYOND™ Phase 2 Trial of Nimacimab for Obesity
Beacon Biosignals, a leader in at-home sleep monitoring and computational neurodiagnostics, today announced a strategic collaboration with Skye Bioscience, Inc. (Nasdaq: SKYE), a pharmaceutical company focused on unlocking new therapeutic pathways for metabolic health, to enhance its upcoming CBEYOND™ Phase 2…
New SPECT Study Unveils Significant Brain Activity Differences in Depression
Researchers in a groundbreaking neuroimaging study have discovered that individuals with depression exhibit significantly higher levels of blood flow in various brain regions. Key findings from the study indicate that depressed patients show increased blood flow in critical brain areas,…
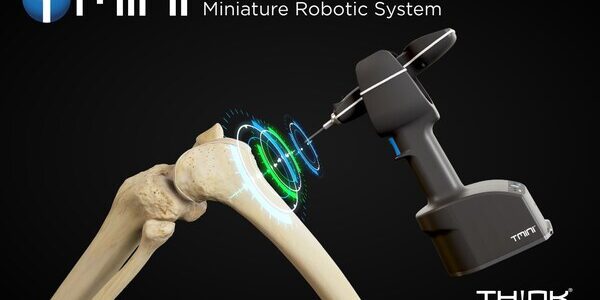
THINK Surgical Receives FDA 510(k) Clearance for TMINI Miniature Robotic System (TMINI 1.1)
THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced that its TMINI® Miniature Robotic System (TMINI 1.1) has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The TMINI 1.1 system software provides substantial…
PDO Max® Recognized as a Top 5 Innovator at Sold Out Medical Aesthetic Show, Modern Beauty Con, For New PDRN-based Skin Booster
PDO Max is thrilled to announce our recent participation and selected recognition in Modern Beauty Con (MBC). At the Modern Beauty Con, held on June 30, 2024, in Boston, the company was selected as one of the top five innovative businesses. Modern…
Vegamour Launches Groundbreaking GRO+ Advanced System to Combat Severe Hair Shedding and Loss
Vegamour, the leader in holistic hair wellness, announces the launch of its latest innovation, the GRO+ Advanced System, dermatologist co-developed and designed to address the concern of severe shedding and hair loss. Launching today, this groundbreaking collection, which includes a Balancing Shampoo, Balancing Conditioner,…