Beckman Coulter’s DxI 9000 Immunoassay Analyzer Honored at Premier, Inc.’s Annual Supplier Innovation Celebration
Beckman Coulter Diagnostics, a clinical diagnostics leader, today announced that the DxI 9000 Immunoassay Analyzer* was honored during Premier, Inc.’s annual supplier Innovation Celebration at its 2024 Breakthroughs Conference and Exhibition. The Innovation Celebration recognizes groundbreaking healthcare technologies that have been…
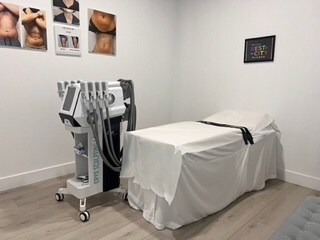
Cryo Sculpting Lab Announces Revolutionary Franchise Opportunity in Non-Invasive Body Contouring
Cryo Sculpting Lab (CSL), a pioneer in non-invasive body contouring, is excited to announce its new franchise opportunity, offering entrepreneurs a chance to own a business in the rapidly growing health and wellness industry. Specializing in cutting-edge fat freezing treatments,…
4WEB Medical Announces Commercial Launch of its Cervical Spine Integrated Plating Solution
4WEB Medical, an orthopedic implant company focused on developing innovative implants that utilize its proprietary Truss Implant Technology™, announced the initial launch of the newest addition to the company’s portfolio, the Cervical Spine Truss System Integrated Plating Solution (CSTS-IPS). The…
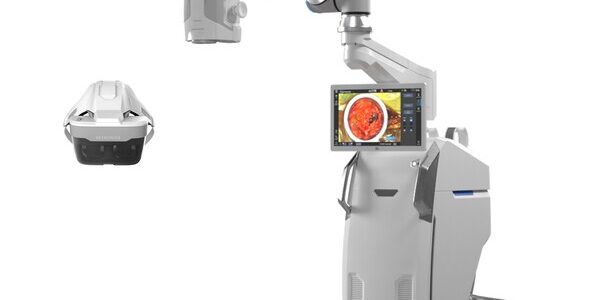
Beyeonics Surgical Announces the First Use of the Beyeonics Maverick System in Surgery
The Beyeonics™ Maverick system has been used in surgery for the first time to successfully give surgeons enhanced visual and operational capabilities during neurosurgical and orthopedic procedures. The headset-based interface uses a state-of-the-art augmented reality visualization system originally developed for F-35…
TOP PMR SPECIALIST DR. AASTIK BHATT JOINS HCAH
HCAH, India’s leading full-stack rehab and geriatric platform today announced a significant development by welcoming Dr Aastik Bhatt, a renowned physiatrist (commonly referred to as a PMR specialist – Physical Medicine and Rehabilitation), to its growing team. Dr Bhatt’s arrival…
PLUSS Advanced Technologies launches Brrf PLUSS Jackets
Pluss Advanced Technologies has launched a cooling wearable technology that can be worn by workers who are outdoors, such as on construction sites, mines, furnaces, shopfloor, in order to prevent heat stress related injuries and fatigue during hot temperatures. A…
MigraKet® Brings the World’s First Medical Food For Migraine to the U.S
International wellness brand Brain Ritual is launching MigraKet®, a revolutionary approach to migraine management developed by renowned neuroscientist and former chronic migraineur, Elena Gross, PhD. With the U.S. release of MigraKet®, sufferers of migraine can anticipate a newfound hope in managing their condition…
AITRICS to Participate in HIMSS USA 2024
AITRICS (CEO Kwang joon Kim), a company specializing in medical artificial intelligence (AI) technology announced on the 11th that it would participate in the ‘HIMSS Global Health Conference & Exhibition’ (2024 HIMSS USA) held in Orlando, Florida, USA from March 11 to 15….
PepGen Announces Clearance of CTA by UK Medicines & Healthcare Products Regulatory Agency to Begin CONNECT2-EDO51, a Phase 2 Clinical Trial designed to support potential accelerated approval of PGN-EDO51 for the Treatment of Duchenne Muscular Dystrophy
PepGen Inc. (Nasdaq: PEPG), a clinical-stage biotechnology company advancing the next generation of oligonucleotide therapies with the goal of transforming the treatment of severe neuromuscular and neurological diseases, today announced that the UK Medicines & Healthcare products Regulatory Agency (MHRA)…
Compass Therapeutics Announces Publication of CTX-8371 Preclinical Data in OncoImmunology, its Bispecific Antibody Checkpoint Inhibitor, now Advancing to First-in-Human Clinical Trial
Compass Therapeutics, Inc. (Nasdaq: CMPX), a clinical-stage, oncology-focused biopharmaceutical company developing proprietary antibody-based therapeutics to treat multiple human diseases, announced the publication of a peer-reviewed article titled, “A bispecific anti-PD-1 and PD-L1 antibody induces PD-1 cleavage and provides enhanced anti-tumor activity” in…