Justrite® Develops Groundbreaking Solution for the Growing Dangers of Lithium-Ion Battery Fires
In response to the critical need for safe storage and charging of lithium-ion batteries, Justrite announces the launch of its state-of-the-art Lithium-Ion Battery Charging Cabinet. Featuring the innovative 9-Layer ChargeGuard™ Containment System, this cutting-edge cabinet is engineered to prevent catastrophic losses…
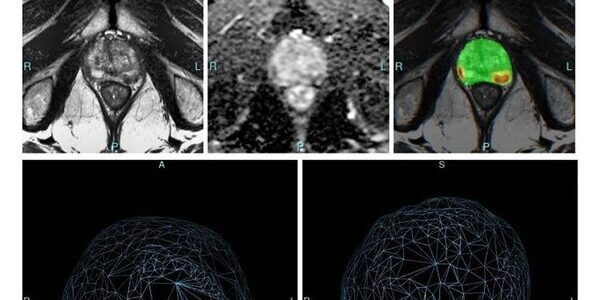
Bot Image, Inc. introduced its Prostate Cancer detection, diagnosis, and screening software at urologist meeting in San Antonio, Texas
Bot Image, Inc., a Nebraska and Maine based Artificial Intelligence medical device company (www.botimageai.com), introduced its Prostate Cancer detection, diagnosis, and screening software to the Urologist community at the recent AUA meeting in San Antonio, Texas and received rave reviews. Urologists from around the world shared…
Penumbra Launches Lightning Flash 2.0 – Latest CAVT Technology Designed to Rapidly Remove Blood Clots
Penumbra, Inc. (NYSE: PEN), the world’s largest thrombectomy company, announced the U.S. Food and Drug Administration (FDA) clearance and launch of Lightning Flash™ 2.0, the next generation computer assisted vacuum thrombectomy (CAVT) system to remove venous thrombus and treat pulmonary…
FDA Grants HemoSonics Expanded Use of its Critical Bleeding Management System with Special 510(k) Clearance
HemoSonics, a medical device company focused on acute bleeding management, today announced it has received Special 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the expanded use of arterial blood samples with its Quantra QStat® Cartridge. HemoSonics’…
NEOS Cranial LOOP™ Receives FDA Clearance for CustomizedBone™ Use
Kelyniam Global (OTC:KLYG) and Fin-ceramica, Faenza S.p.a., makers of custom cranial implants, announced today that the NEOS Surgery Cranial LOOP™ fixation system has received 510(k) clearance from the FDA for use with Finceramica’s CustomizedBone™ hydroxyapatite cranial implant. The Cranial LOOP™ family…
New Knee Research Finds Exactech Technologies Improve Patient Outcomes
Exactech, a developer and producer of innovative implants, instrumentation, and smart technologies for joint replacement surgery, announced new research that reports surgeons using ExactechGPS® guided personalized surgery and its Newton® ligament-driven balancing technique experience clinically significant improvements on postoperative Knee Society…
Mediwave Wins Global Recognition at GLOMO Awards 2024 for Health Technologies for Emergency Response
Mediwave, a leading health technology company, has been recognized as a finalist in the prestigious Global Mobile Awards (GLOMO), part of the Mobile World Congress (MWC) hosted by the GSMA in Barcelona, Spain. This recognition highlights Mediwave’s outstanding contributions in…
Nexalin Technology Commences Sales of Gen-2 Neurostimulation Device to New Mental Health Center in Oman Dedicated to Nexalin’s Technology
Nexalin Technology, Inc. (the “Company” or “Nexalin”) (Nasdaq: NXL; NXLIW) today announced that it has commenced sales of its second generation (Gen-2), 15 milliamp (mA) neurostimulation device into a new mental health center in Oman, dedicated to the use of…
Ocugen, Inc. Announces Dosing Completion of Subjects with Geographic Atrophy in Cohort 1 of Phase 1/2 Clinical Trial Evaluating the Safety and Efficacy of OCU410
Ocugen, Inc. (“Ocugen” or the “Company”) (NASDAQ: OCGN), a biotechnology company focused on discovering, developing, and commercializing novel gene and cell therapies and vaccines, today announced that dosing is complete in the first cohort of its Phase 1/2 ArMaDa clinical…
Beckman Coulter and Fujirebio Expand Partnership to Develop Patient-friendly, Blood-based Neurodegenerative Disease Diagnostics
Beckman Coulter Diagnostics, a clinical diagnostics leader, and Fujirebio, a leader in neurological markers and In Vitro Diagnostic (IVD) manufacturing, today announced an expansion of their partnership focused on development, manufacturing and clinical adoption of neurodegenerative disease assays. In 2023,…