Beacon Biosignals Announces Strategic Collaboration with Skye Bioscience to Integrate Sleep Quality and Sleep Apnea Endpoints in CBEYOND™ Phase 2 Trial of Nimacimab for Obesity
Beacon Biosignals, a leader in at-home sleep monitoring and computational neurodiagnostics, today announced a strategic collaboration with Skye Bioscience, Inc. (Nasdaq: SKYE), a pharmaceutical company focused on unlocking new therapeutic pathways for metabolic health, to enhance its upcoming CBEYOND™ Phase 2…
Wingderm® Shines at IMCAS Asia 2024 with the Innovative Aesthetic Solutions
From June 21 to 23, IMCAS Asia was held in Bangkok, Thailand. Wingderm®, a provider of medical aesthetic devices, has garnered significant attention with its innovative aesthetic solutions. Many visitors stopped by Wingderm®’s booth and stayed for valuable insights. Wingderm® members communicated…
Koning Announces First New York Metropolitan Area Installation of Revolutionary Koning Vera Breast CT at Community Radiology NY
Koning Health, a leader in innovative medical imaging technology, is proud to announce the first installation of its groundbreaking Koning Vera Breast CT system in the New York City metropolitan area at Community Radiology NY on Canal Street. This significant…
Acharya Prashant Engages with IIT Students on Mental Health and Spiritual Well-being
Acharya Prashant, founder of PrashantAdvait Foundation and former civil service officer, interacted with IIT students on mental health. More than 1000 people from across the country participated in the session. He said that there is a deep connection between the…
Beckman Coulter’s DxI 9000 Immunoassay Analyzer Extends Menu with New CE-Marked Hepatitis Assays at ESCMID Global
Beckman Coulter Diagnostics, a leader in clinical diagnostics, announced today, ahead of the congress of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID Global), that it has extended the menu of DxI 9000 Immunoassay Analyzer* assays. Recently, tests…
Pharmactive Presents Affron® Saffron Gummies and Aged Black Garlic
Catering to the world of natural wellness, Pharmactive Biotech Products, SLU will showcase two premier clean-label botanical ingredients: affron® saffron extract and ABG+®, aged black garlic. Both are prominent examples of innovative products designed to improve quality of life. On…
Gedeon Richter: Cariprazine Proves Effective in Treating Dual Diagnosis
New analyses of cariprazine studies were presented by Gedeon Richter Plc. at 32e Annual Congress of the European Psychiatric Association held April 6-9 in Budapest, Hungary. According to scientific posters of the event, cariprazine could be an appropriate treatment not only for…
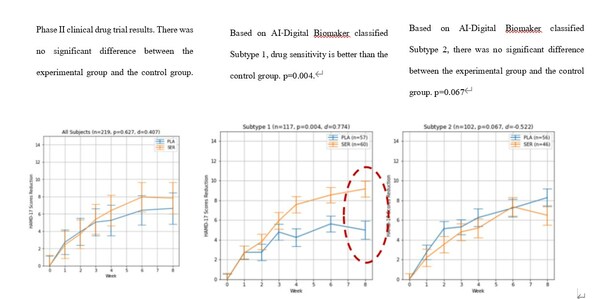
A New Generation of Antidepressant Drug Precision Treatment Based on Revolutionary AI Technology is Born
The9 Limited (Nasdaq: NCTY) (“The9”), an established Internet company, today announced that its investee WM Therapeutics Co., Ltd. (未名脑脑) (“WM Therapeutics”) has achieved revolutionary breakthroughs in the proprietarily developed research and development of antidepressant drug precision treatment. WM Therapeutics’ GenAI multi-dimensional omics…
From U.S. To South Korea: NuBest® Nutrition Expands Global Reach Through Online Retailer Coupang
NuBest® Nutrition proudly announces its global expansion into South Korea through a strategic partnership with Coupang Co., Ltd., the country’s largest online retailer. This milestone marks the introduction of NuBest supplements to the most vibrant market in East Asia, offering…
Acryno To Blends AI and Luxury Concierge Services to Reimagine Healthcare
In an era of rising health costs, increased time to access providers, and growing frustration, it can be difficult for the average person to get the care they need. For those with chronic conditions, these challenges are multiplied exponentially. Acryno…