Beckman Coulter’s DxI 9000 Immunoassay Analyzer Honored at Premier, Inc.’s Annual Supplier Innovation Celebration
Beckman Coulter Diagnostics, a clinical diagnostics leader, today announced that the DxI 9000 Immunoassay Analyzer* was honored during Premier, Inc.’s annual supplier Innovation Celebration at its 2024 Breakthroughs Conference and Exhibition. The Innovation Celebration recognizes groundbreaking healthcare technologies that have been…
Cenegenics Partners With Exoceuticals® to Incorporate Cutting-Edge Exosome-Formulated Skincare Into Holistic Health Practices
Cenegenics, a global leader in personalized healthcare, is partnering with Exoceuticals®, a pioneering skincare company at the forefront of regenerative and exosome-formulated skincare. This collaboration signifies Cenegenics’ unwavering commitment to providing its clients with the most innovative and comprehensive health and…
Penumbra Launches Lightning Flash 2.0 – Latest CAVT Technology Designed to Rapidly Remove Blood Clots
Penumbra, Inc. (NYSE: PEN), the world’s largest thrombectomy company, announced the U.S. Food and Drug Administration (FDA) clearance and launch of Lightning Flash™ 2.0, the next generation computer assisted vacuum thrombectomy (CAVT) system to remove venous thrombus and treat pulmonary…
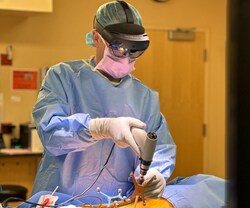
ORTHOINDY ANNOUNCES FIRST SPINE SURGERY UTILIZING SURGICAL THEATER’S XR TECHNOLOGY
OrthoIndy has announced a monumental step forward in surgical innovation by introducing augmented reality (AR) into surgical procedures. Distinguished spine surgeon, Dr. Gregory Poulter, recently performed Indiana’s first-ever surgery utilizing Surgical Theater’s cutting-edge eXperiential Reality (XR) technology. OrthoIndy is one…
Clarius and ThinkSono Introduce a New AI-Guided Ultrasound System Enabling Rapid Assessments of DVTs
Clarius Mobile Health, a leading provider of high-definition handheld ultrasound systems, and ThinkSono, a pioneering medical technology company specializing in ultrasound artificial intelligence (AI) guidance solutions, are introducing a new AI-guided ultrasound system in Europe, which will improve the efficiency…
FDA Grants HemoSonics Expanded Use of its Critical Bleeding Management System with Special 510(k) Clearance
HemoSonics, a medical device company focused on acute bleeding management, today announced it has received Special 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the expanded use of arterial blood samples with its Quantra QStat® Cartridge. HemoSonics’…
HeadaTerm 2: The Most Affordable FDA Cleared Innovative OTC Anti-Migraine Device
WAT Medical Enterprise achieves a major milestone: HeadaTerm 2 receives the OTC clearance from the U.S. Food and Drug Administration (FDA), making it one of the only wearable anti-migraine devices that is available without a prescription in the U.S. Using neuromodulation…
Revive Drip’s Expert IV Nurses Bring Alternative Wellness Solutions to the Greater Puget Sound Area
Revive Drip, a leading mobile intravenous therapy (IV) company providing customized hydration and immune-boosting therapy throughout the Puget Sound, announced today the opening of the company’s brick-and-mortar clinic in Gig Harbor, Washington. The company’s expansion marks an exciting milestone for…
Signia’s Groundbreaking Campaign to Transform Hearing Health Perception
This month marks the launch of Signia’s groundbreaking campaign, aimed at transforming the public’s perception of hearing loss and advocating for widespread ear and hearing care. In line with the “Changing mindsets: Let’s make ear and hearing care a reality…
Reese Pharmaceutical Launches New At-Home Colon Cancer Screening Test
Reese Pharmaceutical is expanding its over-the-counter (OTC) diagnostic portfolio with the launch of ColoTest®, an at-home Fecal Immunochemical Test (FIT) that identifies the presence of hidden blood in the stool in support of the early detection of colon cancer or gastrointestinal disorders…