Kane Biotech Files New Patent on revyve™ Antimicrobial Wound Gel Spray
Kane Biotech Inc. (TSX-V:KNE OTCQB:KNBIF) (the “Company” or “Kane Biotech” or “Kane”) announces that it has filed a patent on its revyve™ Antimicrobial Wound Gel Spray, a follow-on product to its FDA 510(k) cleared revyve™ Antimicrobial Wound Gel, and will…
23andMe Announces FDA Clearance of IND Application for its Dual Mechanism Antibody, 23ME-01473, Targeting ULBP6
23andMe Holding Co. (Nasdaq: ME) (23andMe), a leading human genetics and biopharmaceutical company, announced the U.S. Food and Drug Administration (FDA) has cleared the investigational new drug application for 23ME-01473 (‘1473), a natural killer (NK) cell activator intended to treat…
Outlook Therapeutics® Doses First Subject in NORSE EIGHT
Outlook Therapeutics, Inc. (Nasdaq: OTLK), a biopharmaceutical company working to achieve FDA approval for the first ophthalmic formulation of bevacizumab for the treatment of retinal diseases, today announced that the first subject has been dosed in the NORSE EIGHT clinical…
New Philips Mini TEE ultrasound transducer helps improve cardiac care for more patients
Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced that its latest TEE transducer, designed to serve more patients with improved overall comfort, has received FDA 510(k) clearance. Cardiovascular ultrasound has played a key role in…
Healthcare Leaders Unite for Revolutionary Changes in Upcoming 2024 Budget
As the nation stands on the cusp of the Budget 2024 announcement, leading voices from the healthcare sector have shared their anticipations and aspirations. The sector, which has been at the forefront in the battle against recent health challenges, looks…
Venus Remedies gets coveted GMP certification from Libya
Venus Remedies, a globally recognised pharmaceutical company, has been awarded the Good Manufacturing Practices (GMP) certification for its facilities from the Ministry of Health, Libya. This accomplishment is a testament to the company’s commitment to quality, safety and efficiency in…
Cidara Therapeutics Announces Approval of REZZAYO by the MHRA for the Treatment of Invasive Candidiasis in Adults
Cidara Therapeutics, Inc. (Nasdaq: CDTX), a biotechnology company developing therapies designed to save lives and improve the standard of care for patients facing serious diseases, today announced that the United Kingdom (U.K.) Medicines and Healthcare products Regulatory Agency (MHRA) has…
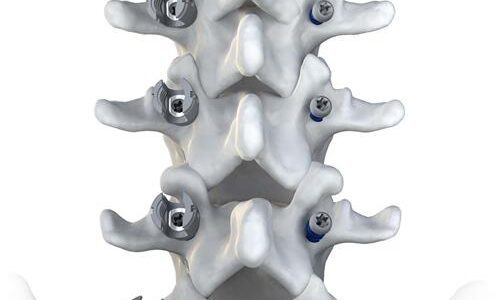
Accelus Introduces LineSider Modular-Cortical System for Posterior Fixation
Accelus, a privately held medical technology company committed to becoming the global market leader in expandable spinal implant technologies, today announced the launch of the LineSider® Modular-Cortical System. Designed for greater procedural visibility, versatility and efficiency, the modular screw design provides…
SCYNEXIS Presented Preclinical Data on Second Generation Fungerp SCY-247 against Mucormycosis at the 11th Advances Against Aspergillosis and Mucormycosis Conference
SCYNEXIS, Inc. (NASDAQ: SCYX), a biotechnology company pioneering innovative medicines to overcome and prevent difficult-to-treat and drug-resistant infections, today announced the presentation of preclinical efficacy data on its second generation fungerp candidate SCY-247. Dr. Ashraf Ibrahim, PhD, FAAM, FECCM, Professor…
Halozyme Announces Takeda Received European Commission Approval for HYQVIA® Co-formulated with ENHANZE® as Maintenance Therapy for Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
Halozyme Therapeutics, Inc. (NASDAQ: HALO) (“Halozyme”) today announced that Takeda received European Commission (EC) approval for HYQVIA® [Immune Globulin Infusion 10% (Human)] co-formulated with Halozyme’s ENHANZE® drug delivery technology as maintenance therapy in patients of all ages with chronic inflammatory demyelinating polyneuropathy (CIDP)…