SMBXL elevates the digital experience for MSMEs by introducing India’s largest B2B and B2C Online Hospital and Medical Supplies Expo
SMBXL, a leading technology company that supports MSMEs, has launched India’s largest ONLINE B2B and B2C Hospital & Medical Supplies Expo. The expo has over 1200+ companies from the Hospital & Medical Supplies sector across India. The participating organisations represent…
HeadaTerm 2: The Most Affordable FDA Cleared Innovative OTC Anti-Migraine Device
WAT Medical Enterprise achieves a major milestone: HeadaTerm 2 receives the OTC clearance from the U.S. Food and Drug Administration (FDA), making it one of the only wearable anti-migraine devices that is available without a prescription in the U.S. Using neuromodulation…
NEOS Cranial LOOP™ Receives FDA Clearance for CustomizedBone™ Use
Kelyniam Global (OTC:KLYG) and Fin-ceramica, Faenza S.p.a., makers of custom cranial implants, announced today that the NEOS Surgery Cranial LOOP™ fixation system has received 510(k) clearance from the FDA for use with Finceramica’s CustomizedBone™ hydroxyapatite cranial implant. The Cranial LOOP™ family…
AITRICS to Participate in HIMSS USA 2024
AITRICS (CEO Kwang joon Kim), a company specializing in medical artificial intelligence (AI) technology announced on the 11th that it would participate in the ‘HIMSS Global Health Conference & Exhibition’ (2024 HIMSS USA) held in Orlando, Florida, USA from March 11 to 15….
Father of Exactech Employee Receives First Ankle Replacement Using Activit-E™ Polyethylene
Exactech Quality Control Manager Wesley Garrett experienced a full circle moment in both his professional and personal life when his father received an Activit-E™ polyethylene insert after following the company’s polyethylene initiatives for six years. In 2018, Garrett was hired as an intern to…
New Models of PENTAX Medical i20c Video Endoscope Series Obtain CE Marks
PENTAX Medical, a division of HOYA Group, have obtained CE marks for new models of PENTAX Medical i20c Video Endoscope Series – PENTAX Medical Video Colonoscope EC34-i20c, PENTAX Medical Video Upper GI Scope EG27-i20c and R/L Knob Adaptor OE-B17. In…
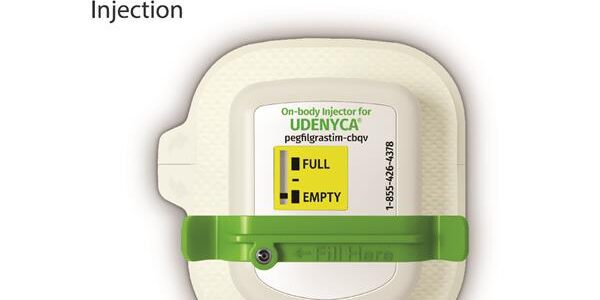
Coherus Announces U.S. Launch of UDENYCA ONBODY™ a Novel and Proprietary State-of-the-Art Delivery System for pegfilgrastim-cbqv
Coherus BioSciences, Inc. (Coherus, NASDAQ: CHRS) today announced the launch of UDENYCA ONBODY™, the company’s on-body injector (OBI) presentation of UDENYCA® (pegfilgrastim-cbqv), is successfully underway, with a broad distribution of accounts nationwide ordering this innovative device, and patients now accessing its benefits….
SIBIONICS Announces Participation in the 17th International Conference on Advanced Technologies and Treatments for Diabetes
SIBIONICS, the world’s third-largest continuous glucose monitoring (CGM) brand, announced that it will participate in the International Diabetes Technology Exhibition at the 17th Symposium on Diabetes at the 17th Symposium on Diabetese International Conference on Advanced Technologies and Treatments for Diabetes…
FDA Advisory Committee Votes in Favor of Abbott’s First-of-Its-Kind TriClip™ System to Treat People With a Leaky Tricuspid Heart Valve
Abbott (NYSE: ABT) today announced that the Circulatory System Devices Panel of the Medical Devices Advisory Committee for the U.S. Food and Drug Administration (FDA) confirmed 13 to 1, with 0 abstention that the benefits of Abbott’s TriClip™ transcatheter edge-to-edge repair…
Coloplast launches Peristeen® Light for people with bowel disorders
The new transanal irrigation device is designed to help people facing defecation issues or stool leakage. It is estimated that 10-15% of the world’s population are affected by chronic constipation1 and that 6% struggle with faecal incontinence2, both of which can…