Hyundai Bioscience Announces Clinical Development Plan for Niclosamide-based Metabolic Anticancer Drug Targeting P53 Mutation Cancer
Hyundai Bioscience announced on April 25th that its clinical development plan of oral “Niclosamide Metabolic Anticancer Drug” targeting cancer patients with intractable cancer caused by p53 gene mutations. Mutations in the p53 gene occur in almost all cancer types and cause intractable…
Sunrise Oncology Centre Introduces Comprehensive Cancer Care in Vasai, a First for Palghar District
Sunrise Oncology Centre, a leading provider of comprehensive cancer care, is proud to announce the opening of its new facility in Vasai, marking the establishment of the first cancer registry in Palghar district. The new centre aims to provide holistic…
RYBREVANT® (amivantamab-vmjw) in Combination With Chemotherapy Is the First FDA Approved Therapy for First-line Treatment of Patients With Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations
Johnson & Johnson (NYSE: JNJ) announced today that following a priority review, the U.S. Food and Drug Administration (FDA) has approved RYBREVANT® (amivantamab-vmjw) in combination with chemotherapy (carboplatin-pemetrexed) for the first-line treatment of patients with locally advanced or metastatic non-small cell lung…
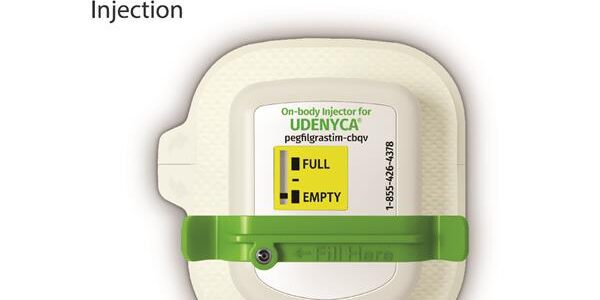
Coherus Announces U.S. Launch of UDENYCA ONBODY™ a Novel and Proprietary State-of-the-Art Delivery System for pegfilgrastim-cbqv
Coherus BioSciences, Inc. (Coherus, NASDAQ: CHRS) today announced the launch of UDENYCA ONBODY™, the company’s on-body injector (OBI) presentation of UDENYCA® (pegfilgrastim-cbqv), is successfully underway, with a broad distribution of accounts nationwide ordering this innovative device, and patients now accessing its benefits….
BioXcel Therapeutics Receives FDA Fast Track Designation for BXCL701 for Treatment of Small Cell Neuroendocrine Prostate Cancer (SCNC)
BioXcel Therapeutics, Inc. (Nasdaq: BTAI), a biopharmaceutical company utilizing artificial intelligence to develop transformative medicines in neuroscience and immuno-oncology, today announced that the U.S. Food and Drug Administration (FDA) has designated as a Fast Track development program the investigation of…
Processa Pharmaceuticals Announces Expansion of NGC-Cap Program into Advanced or Metastatic Breast Cancer
Processa Pharmaceuticals, Inc. (Nasdaq: PCSA) (“Processa” or the “Company”), a clinical-stage pharmaceutical company focused on developing the next generation of chemotherapeutic drugs to improve the efficacy and safety for more patients suffering from cancer, announces it plans to expand the…
MVR-T3011 IV, Global First Intravenous Oncolytic Product, Successfully Concludes Phase I Clinical Study in the U.S. with Outstanding Safety Results
On November 9, 2023, ImmVira announced the successful completion of Phase I clinical study conducted on late-stage patients with various tumor types for our intravenous oncolytic product, MVR-T3011 IV, in the United States. This milestone was marked by the product’s exceptional safety results…
AMGEN PRESENTS NEW LUMAKRAS® (SOTORASIB) PLUS CHEMOTHERAPY DATA IN FIRST-LINE KRAS G12C NSCLC AT WCLC
Amgen (NASDAQ:AMGN) today announced exciting data from a study arm of the CodeBreaK 101 clinical trial, a Phase 1b study evaluating LUMAKRAS® (sotorasib) with carboplatin and pemetrexed in adult patients with KRAS G12C-mutated advanced non-small cell lung cancer (NSCLC). These…
Chemotherapy before surgery reduces risk of colon cancer recurrence, finds Cancer Research UK-funded trial
Administering chemotherapy before surgery can significantly reduce the risk of colon cancer recurrence, according to a Cancer Research UK-funded trial. The FOxTROT trial, led by the University of Birmingham and the University of Leeds, involved 1,053 colon cancer patients from…
6-Month-Old Baby successfully treated for eye cancer at Wadia Hospital, Mumbai
A team headed by Dr Suyash Kulkarni, Head , Interventional Radiology, Bai Jerbai Wadia Hospital For Children successfully performed its 1st ophthalmic artery chemotherapy procedure on a 6-month-old baby boy with eye cancer to save his eyes. Ophthalmic artery chemotherapy…