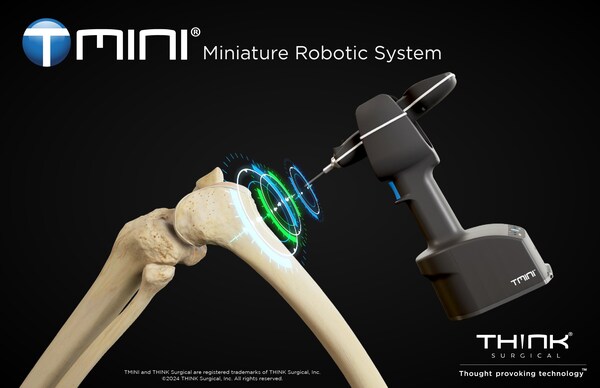
THINK Surgical Receives FDA 510(k) Clearance for TMINI Miniature Robotic System (TMINI 1.1)
THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced that its TMINI® Miniature Robotic System (TMINI 1.1) has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
The TMINI 1.1 system software provides substantial new capabilities empowering surgeon choice throughout total knee arthroplasty procedures. The new TMINI PRO™ workflow, enables Positional Refinement and Optimization of the implant tailored to the patient’s needs. The workflow’s dynamic data capture and real-time feedback allows surgeons to make positional adjustments to help fine-tune implant positioning and stability. The TMINI PRO workflow is intuitive and customizable around surgeon preferences and implant philosophy choices. Surgical plan adjustments are wirelessly transmitted to the TMINI Robot for a seamless surgical experience.
“The new TMINI PRO workflow enabled by the updated TMINI 1.1 software provides me with everything I need from robotics for my total knee replacements,” said Dr. Alexander Sah, Co-Director of the Institute of Joint Restoration. “The added ability to assess real-time soft tissue balance intraoperatively and to adjust my plan according to my preferences in the OR helps me deliver the best possible outcomes for my patients.”
The TMINI System includes a wireless robotic handpiece that assists surgeons in performing total knee replacement. Following a CT based three-dimensional surgical plan which may be updated following intraoperative joint assessment, the TMINI robotic handpiece automatically compensates for surgeon hand movement to locate bone pins along precisely defined planes. Cutting guides are then connected to the bone pins for accurate bone resection. TMINI is easy to use and replaces many of the instruments currently used for manual knee replacement surgery.
“This software release is a major enhancement to our TMINI Robotic System that not only meets our customers’ needs but is a differentiator for our platform in this industry,” said Stuart Simpson, President, and Chief Executive Officer of THINK Surgical. “It is a major accomplishment for our company to receive a 2nd FDA clearance for our TMINI System in less than 15-months. This demonstrates our development capabilities and commitment to innovation as a focused robotics and digital surgery company.”
THINK Surgical is committed to maximizing customer access to its handheld TMINI Miniature Robotic System, including two distinct customer segments, one that prefers an open platform where the customer can choose from a range of implants on the robot and another that prefers an exclusive platform where the customer gains access to the robot in return for loyalty to a single implant brand. THINK Surgical intends to continue adding new implant options to its open implant library over time. The choice of exclusive platform or open implant platform, combined with the robust feature offering and ease of use of the TMINI system should appeal to a broad customer base who may have been resistant to robotics until now.