Dror Ortho-Design, Inc. Launches ZSMILE User Experience Trial
Dror Ortho-Design, Inc. (“Dror” or the “Company”) (OTC PINK: DROR), an AI-based orthodontic platform company that has developed a proprietary solution to correct people’s smiles by straightening teeth using pulsating air delivered by a single custom-made smart aligner, today announced…
Stryker’s Spine Guidance 5 Software featuring Copilot receives 510(k) clearance from FDA
Stryker (NYSE:SYK), a global leader in medical technologies, today announced that its Q Guidance System with Spine Guidance 5 Software featuring Copilot received 510(k) clearance from the U.S. Food and Drug Administration. This first-to-market technology seamlessly integrates smart-powered* instruments into Stryker’s…
Soterix Medical Announces FDA 510(k) Clearance of MEGA-IOM Intraoperative Neuromonitoring System
Soterix Medical Inc., the global leader in stimulation and synergistic monitoring technologies, announced today it has received a 510(k) clearance from the U.S. Food & Drug Administration for its Intraoperative Neurophysiologic Monitoring (IOM) system, MEGA-IOM. The system provides unmatched integrated control…
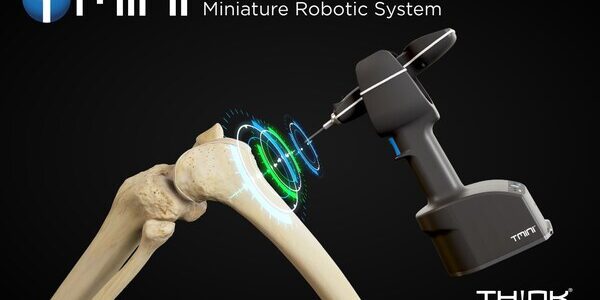
THINK Surgical Receives FDA 510(k) Clearance for TMINI Miniature Robotic System (TMINI 1.1)
THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced that its TMINI® Miniature Robotic System (TMINI 1.1) has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The TMINI 1.1 system software provides substantial…
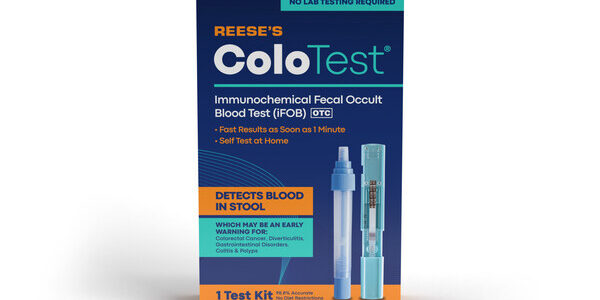
ColoTest for Colon Cancer Screening Now Available at Select Walmart Locations
Reese Pharmaceutical, a privately held manufacturer of over-the-counter (OTC) branded and private label products today announced ColoTest®, its new Fecal Immunochemical Test (FIT) for colon cancer screening, is now available at 3,700 Walmart locations across the U.S. The FDA-cleared ColoTest® identifies the…
Penumbra Launches Lightning Flash 2.0 – Latest CAVT Technology Designed to Rapidly Remove Blood Clots
Penumbra, Inc. (NYSE: PEN), the world’s largest thrombectomy company, announced the U.S. Food and Drug Administration (FDA) clearance and launch of Lightning Flash™ 2.0, the next generation computer assisted vacuum thrombectomy (CAVT) system to remove venous thrombus and treat pulmonary…
DIGITAL THERAPEUTICS STARTUP RESPIREE™ ANNOUNCES INCORPORATION IN US WITH RESIDENCY AT JLABS @ SAN FRANCISCO
Respiree announced today that it has incorporated in the US and will be incubating within Johnson & Johnson Innovation – JLABS @ South San Francisco[1] from February 12, 2024. By expanding footprint within the US, Respiree aims to deepen its relationships and networks…
FDA Grants HemoSonics Expanded Use of its Critical Bleeding Management System with Special 510(k) Clearance
HemoSonics, a medical device company focused on acute bleeding management, today announced it has received Special 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the expanded use of arterial blood samples with its Quantra QStat® Cartridge. HemoSonics’…
DRAWBRIDGE SECURES FDA 510(K) CLEARANCE FOR AT-HOME BLOOD SAMPLING DEVICE NANODROP
Drawbridge Health, a healthcare technology company focused on reinventing the blood draw experience, today announced that its at-home, patented blood sampling device, NanoDrop, has received U.S. Food and Drug Administration (FDA) 510(k) clearance for Over-the-Counter use. The clearance of this…
HeadaTerm 2: The Most Affordable FDA Cleared Innovative OTC Anti-Migraine Device
WAT Medical Enterprise achieves a major milestone: HeadaTerm 2 receives the OTC clearance from the U.S. Food and Drug Administration (FDA), making it one of the only wearable anti-migraine devices that is available without a prescription in the U.S. Using neuromodulation…