DiscGenics Announces US FDA Approval to Proceed with Phase III Clinical Evaluation of Allogeneic, Injectable Disc Progenitor Cell Therapy for Symptomatic Lumbar Degenerative Disc Disease
DiscGenics, Inc., a privately held, late-stage clinical, biopharmaceutical company developing allogeneic, cell-based, regenerative therapies for musculoskeletal degeneration, today announced it has gained acceptance from the U.S. Food and Drug Administration (FDA) for the clinical protocols and Chemistry, Manufacturing, and Controls…
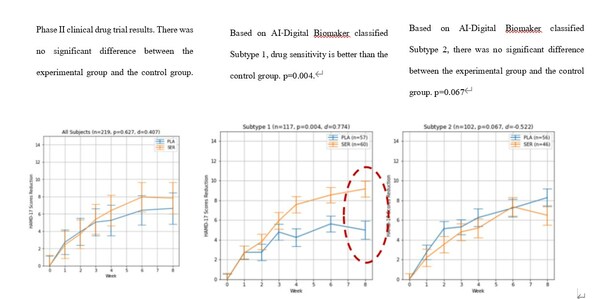
A New Generation of Antidepressant Drug Precision Treatment Based on Revolutionary AI Technology is Born
The9 Limited (Nasdaq: NCTY) (“The9”), an established Internet company, today announced that its investee WM Therapeutics Co., Ltd. (未名脑脑) (“WM Therapeutics”) has achieved revolutionary breakthroughs in the proprietarily developed research and development of antidepressant drug precision treatment. WM Therapeutics’ GenAI multi-dimensional omics…
LyGenesis Announces First Patient Dosed in its Phase 2a Clinical Trial of a First-in-Class Regenerative Cell Therapy for Patients with End-Stage Liver Disease
LyGenesis, a clinical-stage biotechnology company developing cell therapies for large unmet medical needs, announced today that the first patient has been dosed in their Phase 2a clinical trial evaluating their first-in-class allogenic regenerative cell therapy transplanted into patients’ lymph nodes…
Landmark Non-inferiority Clinical Trial Demonstrates Nanodropper’s Positive Impact on Glaucoma Treatment
Nanodropper, Inc., an ophthalmic device company that created the eyedrop volume-reducing Nanodropper® Adaptor, announced today that its clinical trial “An Evaluation of the Efficacy and Safety of Timolol Maleate 0.5% Microdrops Administered with the Nanodropper” was published in the journal Ophthalmology. This was a…
Anavex Life Sciences Initiates Placebo-Controlled U.S. Phase 2 Clinical Trial of ANAVEX®3-71 in Schizophrenia
Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company developing differentiated therapeutics for the treatment of neurodegenerative, neurodevelopmental, and psychiatric disorders, including Alzheimer’s disease, Parkinson’s disease, Rett syndrome, schizophrenia, and other central nervous system (CNS)…
Atrogi Announces Positive Clinical Data from First-in-Class Insulin-Independent Treatment for Type 2 Diabetes
Atrogi, a pioneering clinical-stage biotech company advancing treatments for metabolic disorders, announces the full set of results from its Phase 1 a/b trial with ATR-258, its first-in-class treatment for type 2 diabetes (T2D). This clinical trial, conducted on both healthy…
Recce Pharmaceuticals Doses Next Cohort in Phase I/II Trial of RECCE® 327 for Urinary Tract Infections and Urosepsis
Recce Pharmaceuticals Ltd (ASX: RCE, FSE: R9Q), (the Company), the Company developing a new class of synthetic anti-infectives, today announced it dosed the next cohort of healthy subjects at 3,000mg within a fast infusion rate of 20-minutes in a Phase…
Ocugen, Inc. Announces Dosing Completion of Subjects with Geographic Atrophy in Cohort 1 of Phase 1/2 Clinical Trial Evaluating the Safety and Efficacy of OCU410
Ocugen, Inc. (“Ocugen” or the “Company”) (NASDAQ: OCGN), a biotechnology company focused on discovering, developing, and commercializing novel gene and cell therapies and vaccines, today announced that dosing is complete in the first cohort of its Phase 1/2 ArMaDa clinical…
Arctic Therapeutics and Nacuity Pharmaceuticals Announce European Medicines Agency Approval to Initiate First Clinical Trial of AT-001 (NPI-001) for the Treatment of HCCAA
Arctic Therapeutics ehf, an Icelandic drug development company and partner Nacuity Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company developing treatments for retinitis pigmentosa, cataracts and other diseases caused by oxidative stress, today announced approval by the European Medicines Agency (EMA) for…
First Patient Treated in Phase 2 GvHD Trial
Cynata Therapeutics Limited (ASX: “CYP”, “Cynata”, or the “Company”), a clinical-stage biotechnology company specialising in cell therapeutics, confirms that the first patient has been enrolled and treated in its Phase 2 clinical trial of CYP-001 in high-risk acute graft versus host disease (aGvHD). CYP-001 is…