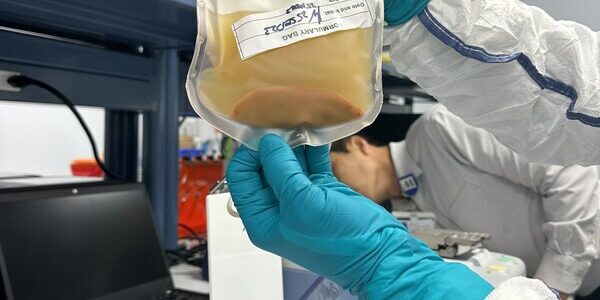
LyGenesis Announces First Patient Dosed in its Phase 2a Clinical Trial of a First-in-Class Regenerative Cell Therapy for Patients with End-Stage Liver Disease
LyGenesis, a clinical-stage biotechnology company developing cell therapies for large unmet medical needs, announced today that the first patient has been dosed in their Phase 2a clinical trial evaluating their first-in-class allogenic regenerative cell therapy transplanted into patients’ lymph nodes…
European Medicines Agency Validates Type II Variation for Astellas’ XTANDI® (enzalutamide) for Treatment of Non-Metastatic Hormone-Sensitive Prostate Cancer with High-Risk Biochemical Recurrence
Astellas Pharma Inc. (TSE: 4503, President and CEO: Naoki Okamura, “Astellas”) today announced that the European Medicines Agency (EMA) has validated its Type II variation for XTANDI® (enzalutamide) for the treatment of patients with non-metastatic hormone-sensitive prostate cancer (nmHSPC; also…