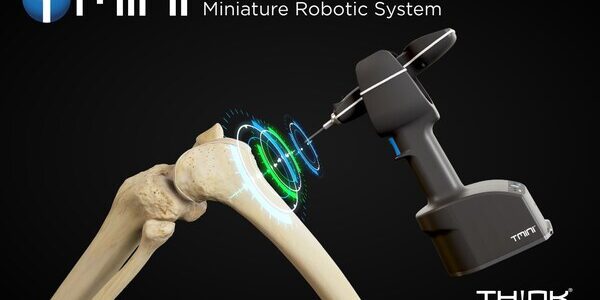
THINK Surgical Receives FDA 510(k) Clearance for TMINI Miniature Robotic System (TMINI 1.1)
THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced that its TMINI® Miniature Robotic System (TMINI 1.1) has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The TMINI 1.1 system software provides substantial…
Vitestro starts largest-scale clinical trial globally for its autonomous blood drawing device, with enrolment of first patients in A.D.O.P.T.
Vitestro, expert in medical robotics, has launched the A.D.O.P.T. Trial for its autonomous blood drawing device (image). With an anticipated sample size of over 10,000 patients, this is the most significant study globally to date of autonomous blood collection. Vitestro…