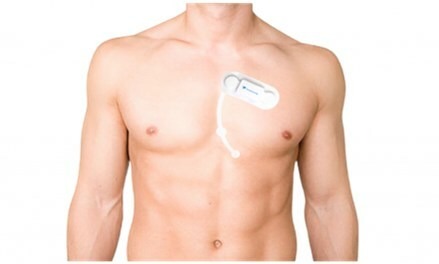
SmartCardia Receives FDA Clearance for its 14-Day 7-Lead ECG Real-Time Patch and Cloud Platform
SmartCardia has received FDA clearance for its 7-lead real-time ECG monitoring patch and cloud platform. SmartCardia’s 7L patch is easy-to-wear, cable-free, waterproof and can be used for continuous monitoring for up to 14 days. “SmartCardia’s 7L patch and cloud platform…
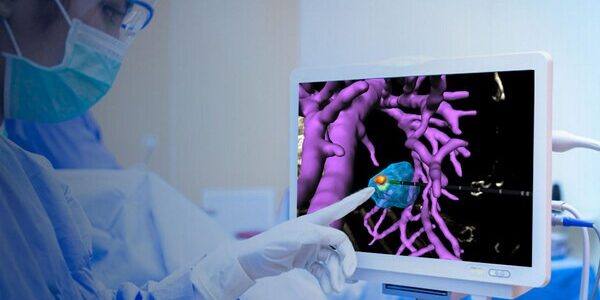
Techsomed Announces FDA Clearance for Ablation Treatment Planning and Confirmation Software
Techsomed Ltd., a medical software innovator dedicated to enhancing clinical impact in ablation therapy, announced today that it has received 510(k) clearance from the USA Food and Drug Administration (FDA) for its VisAble.IO software intended to assist physicians in planning…
First AI-Powered Precision Oncology Platform for Prostate Cancer Care, iQuestTM, Receives FDA Clearance
Avenda Health, an AI healthcare company creating the future of personalized prostate cancer care, today announced its cancer management platform, iQuestTM, has received 510(k) clearance by the U.S. Food and Drug Administration (FDA). iQuest combines existing patient-specific diagnostic information and…